INTRODUCTION
Cancer remains one of the most significant challenges in modern medicine, affecting millions of lives globally each year. , with an estimated 1.9 million new cases and 609,360 deaths in the United States in 2022 alone, according to the American Cancer Society's Cancer Facts & Figures 2022 report [1]. his complex disease encompasses a wide variety of types, each with its own unique characteristics and treatment challenges.
While traditional treatments such as surgery, chemotherapy, and radiation therapy have made significant strides in improving survival rates, they often come with substantial side effects and limitations. In recent years, the landscape of cancer treatment has been revolutionized by the advent of immunotherapies. These innovative treatments harness the body's own immune system to recognize and combat cancer cells more effectively.
The growing enthusiasm around cancer immunotherapies is well-founded, as they offer the promise of more targeted and durable responses compared to conventional therapies. We will explore the principles, successes, challenges, and future perspectives of cancer immunotherapies, highlighting their potential to transform cancer treatment.
Main Classes of Cancer Immunotherapies
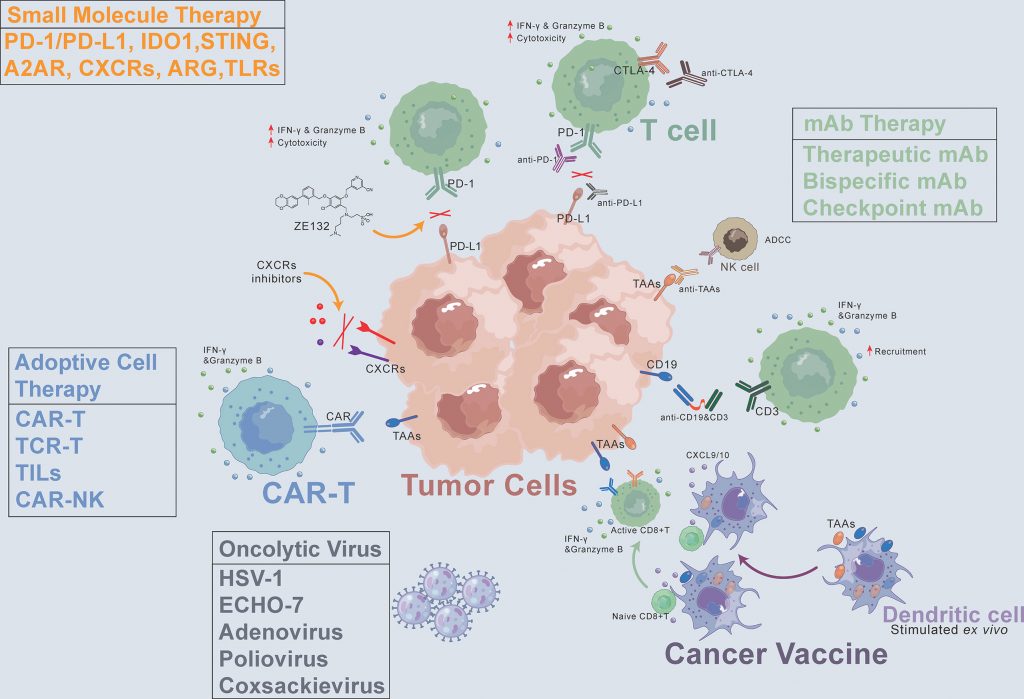
Pardoll's article describes three main classes of immunotherapies that are revolutionizing cancer treatment: immune checkpoint inhibitors, cellular therapies, and therapeutic vaccines 2.
- Immune checkpoint inhibitors, such as anti-CTLA-4 (ipilimumab) and anti-PD-1/PD-L1 antibodies (nivolumab, pembrolizumab), block inhibitory pathways of T lymphocytes to restore their antitumor activity. These therapies have been approved for various cancers, including melanoma.
- Cellular therapies, like CAR-T cells, genetically modify T lymphocytes to specifically target tumor cells. Treatments like tisagenlecleucel and axicabtagene ciloleucel are approved for lymphomas and leukemias.
- Therapeutic vaccines stimulate the immune system against tumor antigens, with sipuleucel-T approved for prostate cancer.
To illustrate the classes described by Pardoll, Haanen et al.'s provide concrete examples of approved cancer immunotherapies, highlighting their clinical successes and significant impact [3].
For example, ipilimumab is approved for melanoma, while anti-PD-1 antibodies like nivolumab and pembrolizumab are used in several cancers. Cellular therapies, such as CAR-T cells targeting CD19, are approved for lymphomas and leukemias. Regarding therapeutic vaccines, sipuleucel-T is approved for prostate cancer, and agents like interferon α and interleukin-2 are used in melanoma treatment.
These concrete examples of clinically validated immunotherapies demonstrate the progress made and pave the way for future advancements, with ongoing clinical trials exploring new targets and strategies to enhance efficacy and overcome resistance.
Promising results
Among others, Immune checkpoint inhibitors, such as anti-PD-1 and anti-CTLA-4 antibodies, have shown promising results in terms of efficacy and survival in clinical trials, heralding a new era in cancer treatment. A pivotal phase 3 study, CheckMate 067,evaluated the combination of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) in advanced melanoma [4]. The results of this trial were encouraging, with a median overall survival of 11.5 months in the combination arm compared to 6.9 months with ipilimumab alone. Additionally, the 3-year overall survival rate was 58% for the combination therapy versus 34% for ipilimumab alone. Objective response rates were also significantly higher, at 58% with the combination therapy compared to 19% with ipilimumab alone.
These findings underscore the benefit of combining treatments that target PD-1 and CTLA-4 to enhance antitumor efficacy, paving the way for new, promising therapeutic strategies for cancer patients. As we look to the future of cancer treatment, these results highlight the potential of immune checkpoint inhibitors to significantly improve patient outcomes and redefine the standards of cancer care.
However, it is important to note that these promising results are accompanied by observed limitations.
Observed limitations
While promising, immune checkpoint inhibitors also present limitations that have been highlighted in clinical trials.
One major concern is the increased toxicities associated with the excessive activation of the immune system. The study by Wolchok et al. reveals that the combination of ipilimumab and nivolumab results in a higher incidence of grade 3-4 adverse effects (59%) compared to nivolumab alone (21%) [5]. The main immune-related toxicities include colitis, hepatitis, skin disorders, endocrine disorders, and pulmonary issues.
Additionally, a significant proportion of patients exhibit primary resistance or develop secondary resistance to anti-PD-1 and anti-CTLA-4 therapies. The mechanisms behind these resistances often involve mutations in the IFN-γ pathway or deficiencies in antigen presentation.
Despite the clinical success of these treatments, understanding and managing these toxicities, as well as developing strategies to prevent resistances, remain crucial challenges in optimizing the use of immunotherapies.
Addressing these limitations is essential as we continue to explore the future of cancer treatment and seek to improve the efficacy and safety of immunotherapies for a broader range of patients.
Future perspectives
Cancer immunotherapies are poised to continue their development and improvement in the coming years. One major research focus is the optimization of CAR-T cell therapies. According to Neelapu et al.), scientists are working on enhancing the in vivo persistence of CAR-T cells, targeting new tumor antigens, using memory T cells, and combining these treatments with checkpoint inhibitors [6].
These efforts aim to improve the efficacy and durability of CAR-T cell therapies, making them more effective for a broader range of cancers.
The goal is to develop therapeutic strategies that combine multiple immunotherapies synergistically, potentially overcoming current limitations and resistances.
In addition to optimizing existing therapies, researchers are exploring new immunomodulatory targets beyond CTLA-4 and PD-1.
Sharma and Allison highlight emerging targets such as LAG3, TIM3, and the enzyme IDO [7]. LAG3 and TIM3 are immune receptors that regulate T cell activation, showing promise when combined with PD-1 inhibitors. IDO is an immunosuppressive enzyme, with inhibitors being tested in combination therapies. Targeting these molecules could enhance the effectiveness of immunotherapy by overcoming current limitations and resistances, offering new strategies for cancer treatment.
These promising perspectives indicate a bright future for cancer immunotherapies, with ongoing research likely to yield more effective and safer treatment options for patients.
CONCLUSION
The future of cancer treatment is increasingly intertwined with the advancements in immunotherapy. As highlighted, the progress in immune checkpoint inhibitors, cellular therapies, and therapeutic vaccines marks a significant shift towards more targeted and durable cancer treatments. Clinical trials have shown promising results, demonstrating enhanced efficacy and survival rates, which are crucial steps forward in the fight against cancer.
However, the journey is not without challenges. The observed toxicities and resistances highlight the need for ongoing research to optimize these therapies and manage their side effects. Looking ahead, the optimization of CAR-T cell therapies and the exploration of new immunomodulatory targets present exciting avenues for further advancements. By addressing current limitations and developing synergistic therapeutic strategies, we can unlock the full potential of immunotherapies.
The continued innovation and dedication in this field hold the promise of transforming cancer treatment, offering hope for improved patient outcomes and paving the way for a future where cancer can be more effectively controlled.
REFERENCES
[2] https://www.nature.com/articles/nrc3239
[3] https://www.nature.com/articles/nrclinonc.2016.203
[4] https://www.nejm.org/doi/full/10.1056/nejmoa1809697
[5] https://www.nejm.org/doi/full/10.1056/NEJMoa1703643